Research
New Finding Realizes Ambient Electrosynthesis of Urea with Nitrate and Carbon Dioxide over Iron-Based Dual-Sites
| By
Urea (CO(NH2)2) has been applied both in agricultural and pharmaceutical field. The Bosch-Meiser process widely used now results in high energy consumption and CO2 emission. Therefore, it is imperative to explore energy-saving and economical routes for urea synthesis under mild conditions.
However, the electrosynthesis of urea with CO2 and NO3- under ambient conditions,which is an efficient way, is still far away from application. This is because the key step needs an efficient electrocatalyst enabling adsorption and activation of NO3- and CO2 to accomplish the C-N coupling.
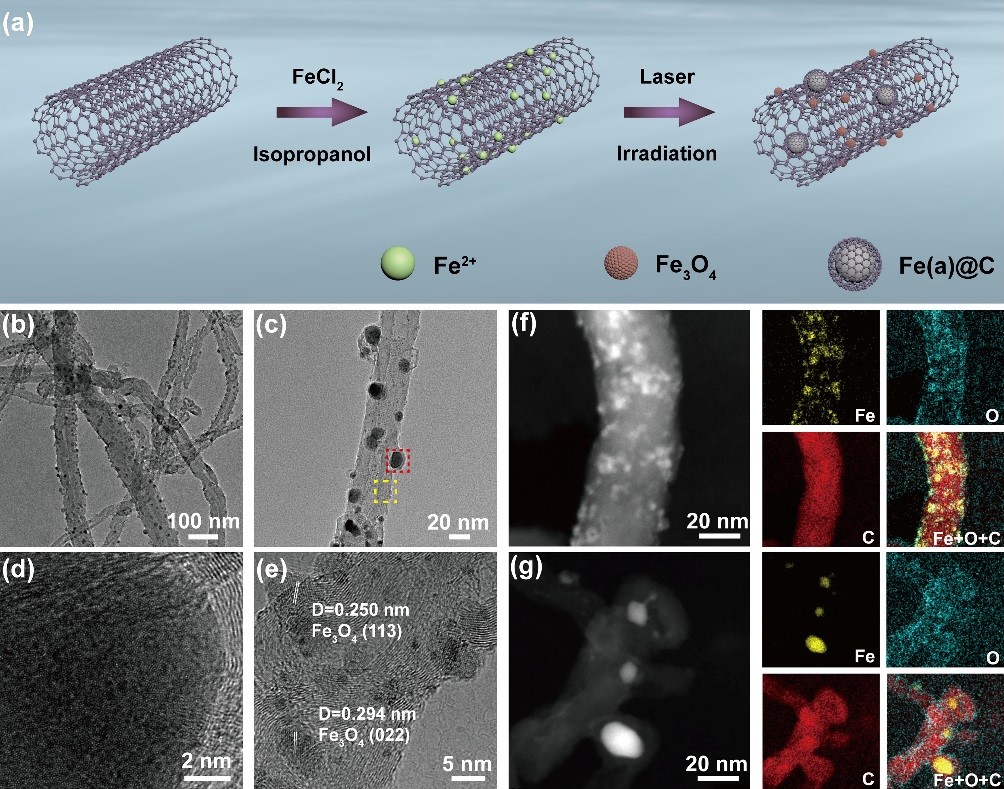
Researchers from Hefei Institutes of Physical Science of Chinese Academy of Sciences has now developed a liquid-phase laser irradiation route to fabricate symbiotic carbon encapsulated amorphous iron (Fe(a)@C) and iron oxide nanoparticles (Fe3O4 NPs) on carbon nanotubes (denoted as Fe(a)@C-Fe3O4/CNTs).
The as-fabricated Fe(a)@C-Fe3O4/CNTs contained two Fe-based active components, namely, Fe@C NPs with the particle sizes of 10~20 nm and Fe3O4 NPs with the particle sizes of 1~5 nm.
The presence of two different structural units in Fe(a)@C-Fe3O4/CNTs made it possible to synergistically electrocatalytic activate CO2 and NO3- to realize the C-N coupling for urea synthesis.
As expected, Fe(a)@C-Fe3O4/CNTs exhibited superior activity toward the electrocatalytic coupling of CO2 and NO3- for urea synthesis, affording a urea yield of 1341.3±112.6 μg h-1 mgcat-1 and a faradic efficiency (FE) of 16.5±6.1% at -0.65 V (vs. RHE) in 0.1 M KNO3 electrolyte.
Both experimental and theoretical results unveiled that Fe(a)@C was mainly responsible for the electrocatalytic reduction of NO3- to form *NH2 intermediates, while Fe3O4 was more beneficial for the electrocatalytic reduction of CO2 to form *CO intermediates.
The synergistically catalytic effect contributes the excellent electrocatalytic performance of urea synthesis at ambient conditions.
This work was financially supported by the Natural Science Foundation of China.
- Attachments Download:
-
contact
Prof. ZHANG Haimin
E-mail: zhanghm@issp.ac.cn


