Research
Interfacial Engineering Boosts the Electrocatalytic Ammonia Synthesis of Rhodium Catalyst
| By
A research team led by Prof. ZHANG Haimin from the Institute of Solid State Physics, Hefei Institutes of Physical Science (HFIPS) of Chinese Academy of Sciences (CAS) reported their finding of dodecanethiol-modified metallic rhodium (Rh) for high-performance electrocatalytic nitrogen (N2) to ammonia (NRR).
The interfacial engineering approach they applied in this study, according to the team, is much helpful for developing high-efficiency NRR electrocatalysts to synthesize ammonia under ambient conditions.
The relevant result was published in Nano Research.
Compared with the Haber-Bosch process of ammonia synthesis with harsh reaction conditions and high energy consumption, the electrocatalytic NRR could be carried out at room temperature and pressure, and water was the source of hydrogen. Therefore, it is of great scientific research value and industrial application feasibility.
However, the non-dipole and low solubility of nitrogen make it difficult to adsorb on the catalyst surface and be activated. In addition, electrolytes are a natural proton source. Compared with N2, the protons generated by water splitting have lower activation energy, so the reaction sites are more easily occupied by protons. The number of active sites for NRR was reduced, resulting in a lower ammonia yield rate.
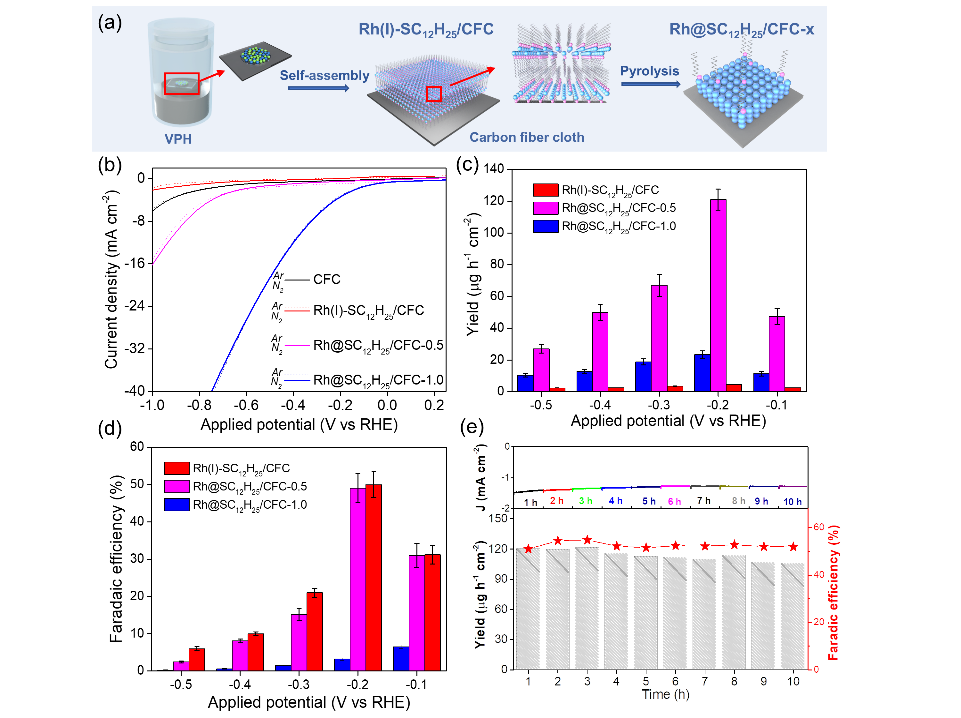
In this research, the dodecanethiol-modified Rh was fabricated via a facile saturated dodecanethiol vapor-phase hydrothermal reaction followed by a low-temperature pyrolysis process. The hydrophobic dodecanethiol molecules on the surface of Rh created a stereo-hindrance effect, which inhibited the diffusion of water molecules or H+ to the metal surface and facilitates N2 adsorption, thus improving the NRR selectivity.
Furthermore, density-functional-theory calculations unveiled that the surface hydrogen (H*) coverage and the NRR reaction energy barrier were both decreased after dodecanethiol modification, thereby greatly enhancing the NRR performance.
This research provided new insights into the effect of the metal-organic interface and H* coverage on the electrochemical NRR activity.
This work was supported by the This work was financially supported by the Natural Science Foundation of China (grant no. 51872292), the young project of Anhui Provincial Natural Science Foundation (grant no. 1908085QB83).
- Attachments Download:
-
contact
Prof. ZHANG Haimin
E-mail: zhanghm@issp.ac.cn


