Research
New Approach Developed for Electrocatalytic H2O2 Production and Biomass Upgrading
| By
Scientists from Institute of Solid State Physics, Hefei Institutes of Physical Science (HFIPS) of Chinese Academy of Sciences (CAS) have synthesized an oxygen-coordinated Fe single atoms and atom clusters catalyst, manifesting superior electrocatalytic performance toward H2O2 production and biomass upgrading.
Hydrogen peroxide (H2O2) is a widely used chemical with applications in diverse fields such as environment, energy, and healthcare. While traditionally manufactured through energy-intensive processes, electrocatalytic synthesis offers a greener and more efficient method using water and oxygen. However, this approach requires advanced electrocatalysts for high yield and selective H2O2 production, and further attention is needed for utilizing the generated H2O2, particularly in electrochemical organic oxidation processes. This presents significant potential for value-added applications beyond environmental remediation.
In this study, the scientists utilized bacterial cellulose as the adsorption regulator and carbon source in combination with a multi-step approach involving wet-chemistry impregnation, pyrolysis, and acid-etching processes to create a catalyst termed FeSAs/ACs-BCC, consisting of oxygen-coordinated Fe single atoms and atom clusters. The presence of both Fe single atoms and clusters was confirmed using advanced imaging techniques such as aberration-corrected scanning transmission electron microscopy. Also, the atomic structure of Fe was determined using X-ray fine structure absorption spectroscopy and X-ray photoelectron spectroscopy.
This catalyst demonstrated outstanding electrocatalytic performance and selectivity for the 2-electron oxygen reduction reaction (2e- ORR) under alkaline conditions. Further H-cell experiments confirmed the accumulation of H2O2 in the electrolyte.
Researchers successfully coupled the in situ generated H2O2 with the electro-Fenton process using ethylene glycol as the reactant and acidified 0.1M Na2SO4 as the electrolyte. This led to a high rate of ethylene glycol conversion and high selectivity for formic acid, showing that the electro-Fenton process has the potential to improve biomass-derived feedstocks through oxidative upgrading.
Aside from that, they developed a three-phase flow cell based on the gas diffusion electrode to further enhance the H2O2 yield.
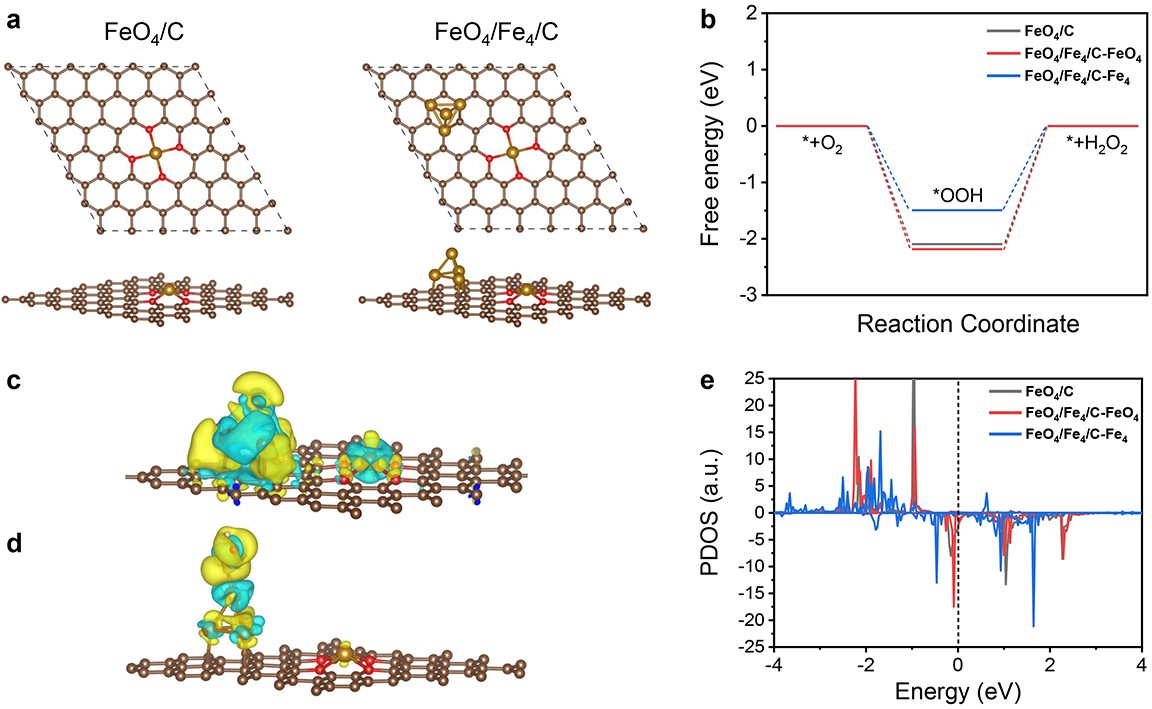
Density functional theory analyses indicated that the actual catalytically active sites in the 2e- ORR process were the Fe clusters, and the electronic interaction between Fe single atoms and Fe clusters could significantly enhance the electrocatalytic performance toward 2e- ORR.
This work would be helpful for design and development of atomic level electrocatalysts for high-efficiency 2e- ORR to H2O2 and biomass upgrading.
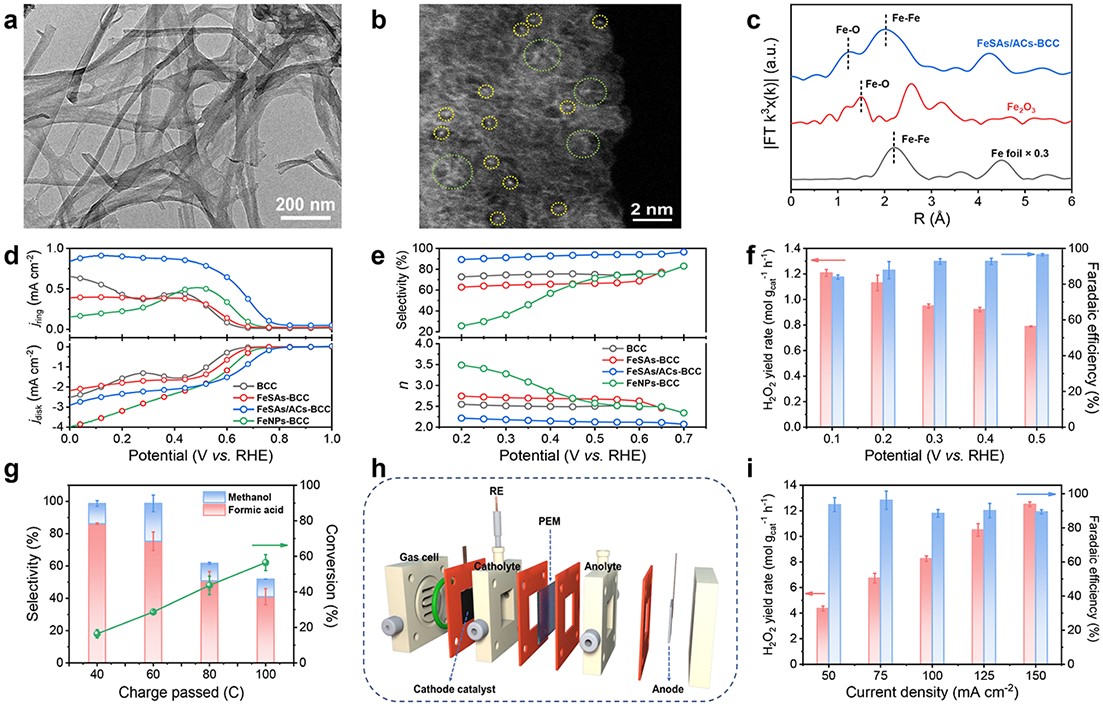
Fig. 1 Characterizations, electrochemical H2O2 synthesis performance and coupled electro-Fenton process of FeSAs/ACs-BCC. (Image by XU Hui)
- Attachments Download:
-
contact
Prof. ZHANG Haimin
E-mail: zhanghm@issp.ac.cn


